


Health Canada approved the Pfizer-BioNTech vaccine for kids 5-11 today. But NACI’s recommendation for dose intervals is different from Health Canada’s approval. What’s up with that?
Health Canada authorizes vaccines based on clinical trial data. Pfizer tested the vaccines with 3 week intervals, so that’s what Health Canada approved. ✅
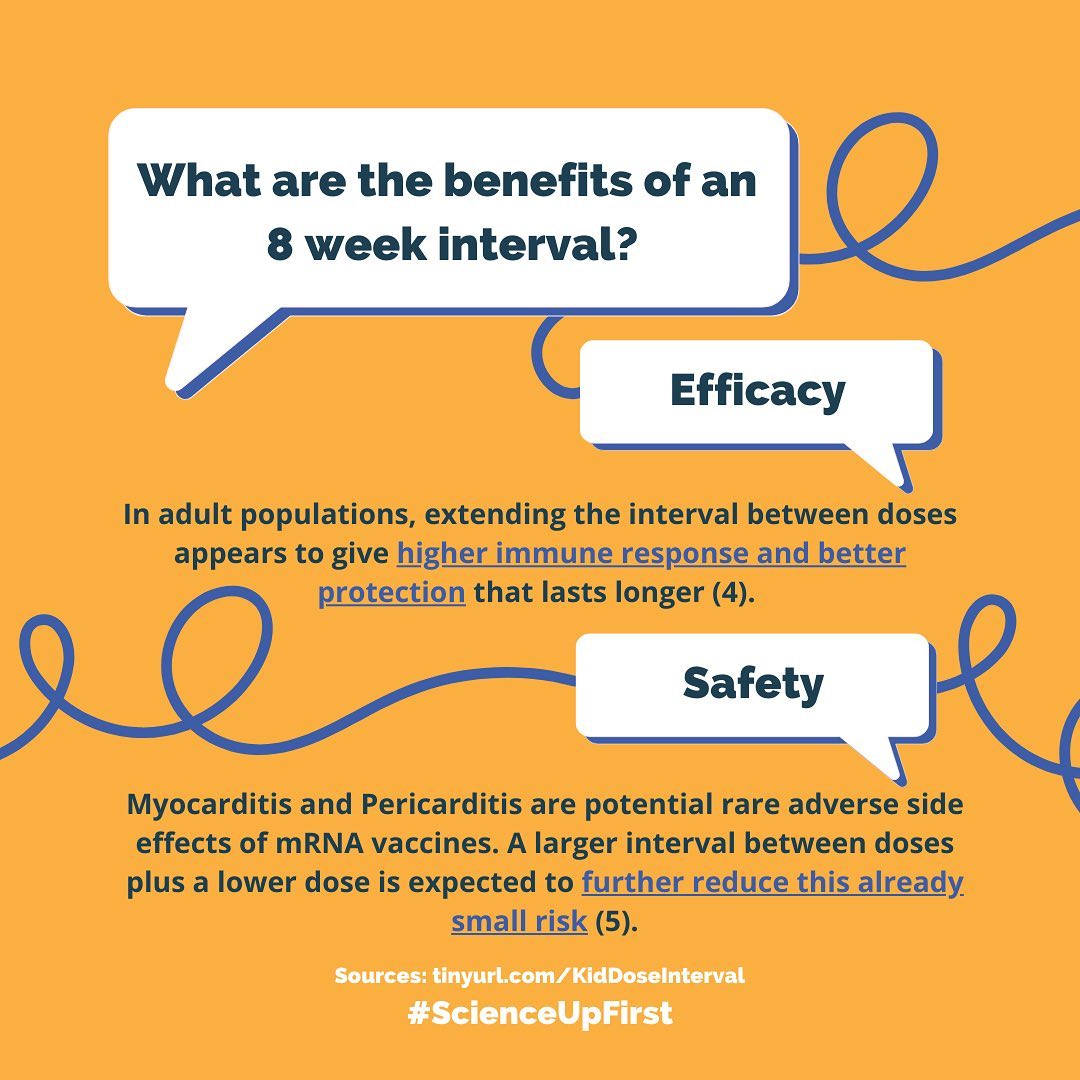
NACI recommends how best to use the authorized vaccine. An interval of at least 8 weeks may contribute added efficacy and safety on an already very safe, effective and high-quality vaccine.
Read through for more information on the different recommendations for dose intervals!
Share our original Tweet!
Health Canada approved the Pfizer-BioNTech vaccine for kids 5-11 today.
NACI has also provided recommendations on how best to use the vaccines, including dose interval.
Let’s discuss why they recommend at least 8 weeks between doses.
[1/7]#ScienceUpFirst pic.twitter.com/cgXcvvjA6c
— ScienceUpFirst | LaScienced’Abord (@ScienceUpFirst) November 19, 2021
View our original Instagram Post!
View this post on Instagram
- Health Canada authorizes use of Comirnaty (the Pfizer-BioNTech COVID-19 vaccine) in children 5 to 11 years of age
- Santé Canada autorise l’utilisation du vaccin Comirnaty (vaccin contre la COVID-19 de Pfizer-BioNTech) chez les enfants de 5 à 11 ans
- An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Recommendation on the use of the Pfizer-BioNTech COVID-19 vaccine (10 mcg) in children 5-11 years of age
- CCNI Recommandation sur l’utilisation du vaccin contre la COVID-19 de Pfizer-BioNTech (10 mcg) chez les enfants âgés de 5 à 11 ans
- Joint Committee on Vaccination and Immunisation (JCVI) advice on COVID-19 vaccination in people aged 16 to 17 years: 15 November 2021
- Summary of National Advisory Committee on Immunization (NACI) statement: Recommendations on the use of COVID-19 vaccines
- An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Recommendation on the use of the Pfizer-BioNTech COVID-19 vaccine (10 mcg) in children 5-11 years of age
- CCNI Recommandation sur l’utilisation du vaccin contre la COVID-19 de Pfizer-BioNTech (10 mcg) chez les enfants âgés de 5 à 11 ans




