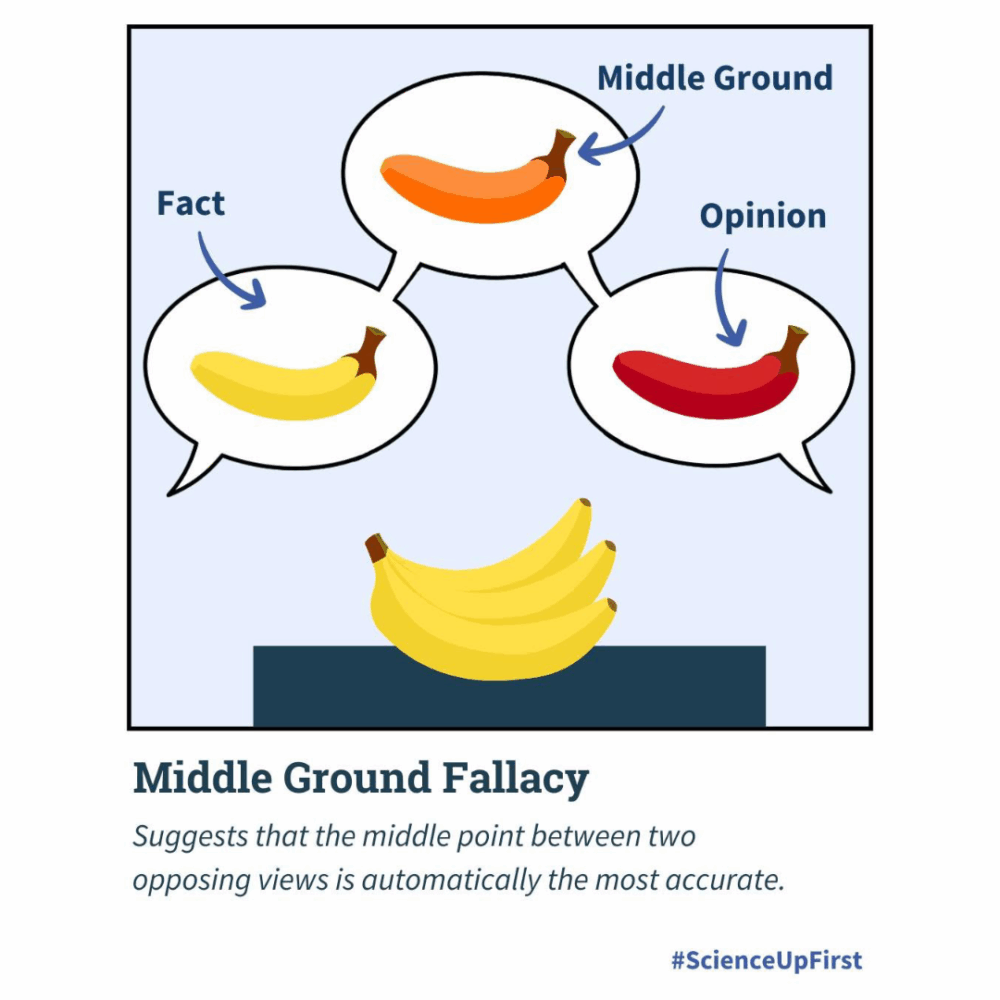
Public commentary in response to the vaccine advisory groups’ meeting with the US Food and Drug Administration over the Emergency Use Authorization (EUA) of the COVID-19 vaccine in children (5-11) displayed overwhelming opposition to the EUA and vaccine-risk misperceptions. (April 19, 2024)
Related Posts
Misinformation 101
Developing critical thinking and decision-making skills for cancer information: the Informed Health Choice-Cancer online learning resource
November 2 2025