



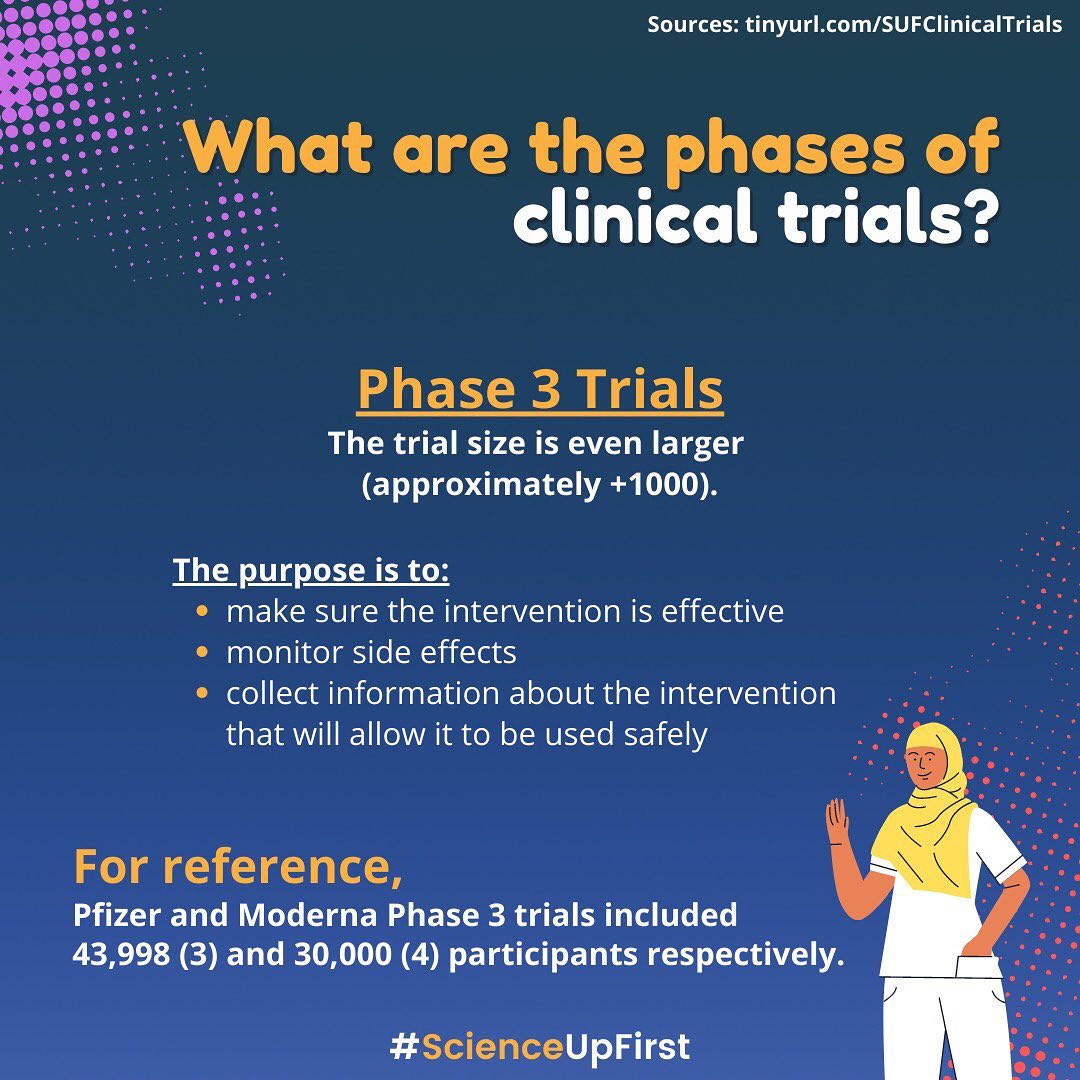


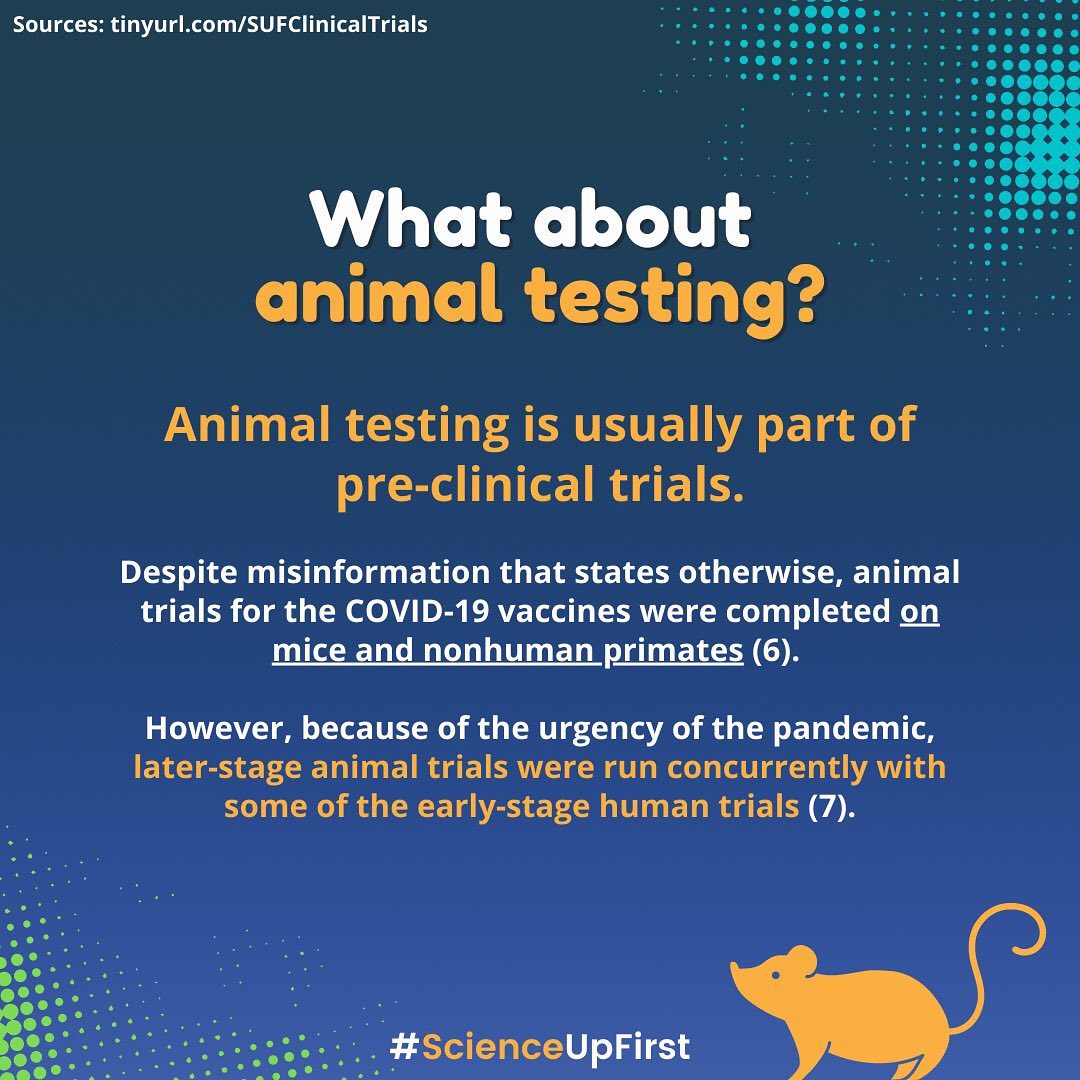

What exactly is a clinical trial and how do they work?
We’ve heard a lot of misinformation about the COVID-19 vaccine clinical trials. We’re here to clear things up
Share our original Tweet!
We’ve heard a lot of misinformation about the COVID-19 vaccine clinical trials.
So what exactly is a clinical trial and how do they work?#ScienceUpFirst
[1/13] pic.twitter.com/hd7mnaggy5
— ScienceUpFirst | LaScienced’Abord (@ScienceUpFirst) March 9, 2022
View our original Instagram Post!
View this post on Instagram
Sources
- The Basics | National Institutes of Health (NIH)
- Clinical trials and drug safety – Canada.ca
- Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals – Full Text View – ClinicalTrials.gov
- A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19 – Full Text View – ClinicalTrials.gov
- Fact check: It is standard practice for vaccine safety monitoring to continue after approval | Reuters
- Fact Check-COVID-19 vaccines did not skip animal trials because of animal deaths | Reuters
- Pfizer and Moderna did not skip animal trials | AP News




