
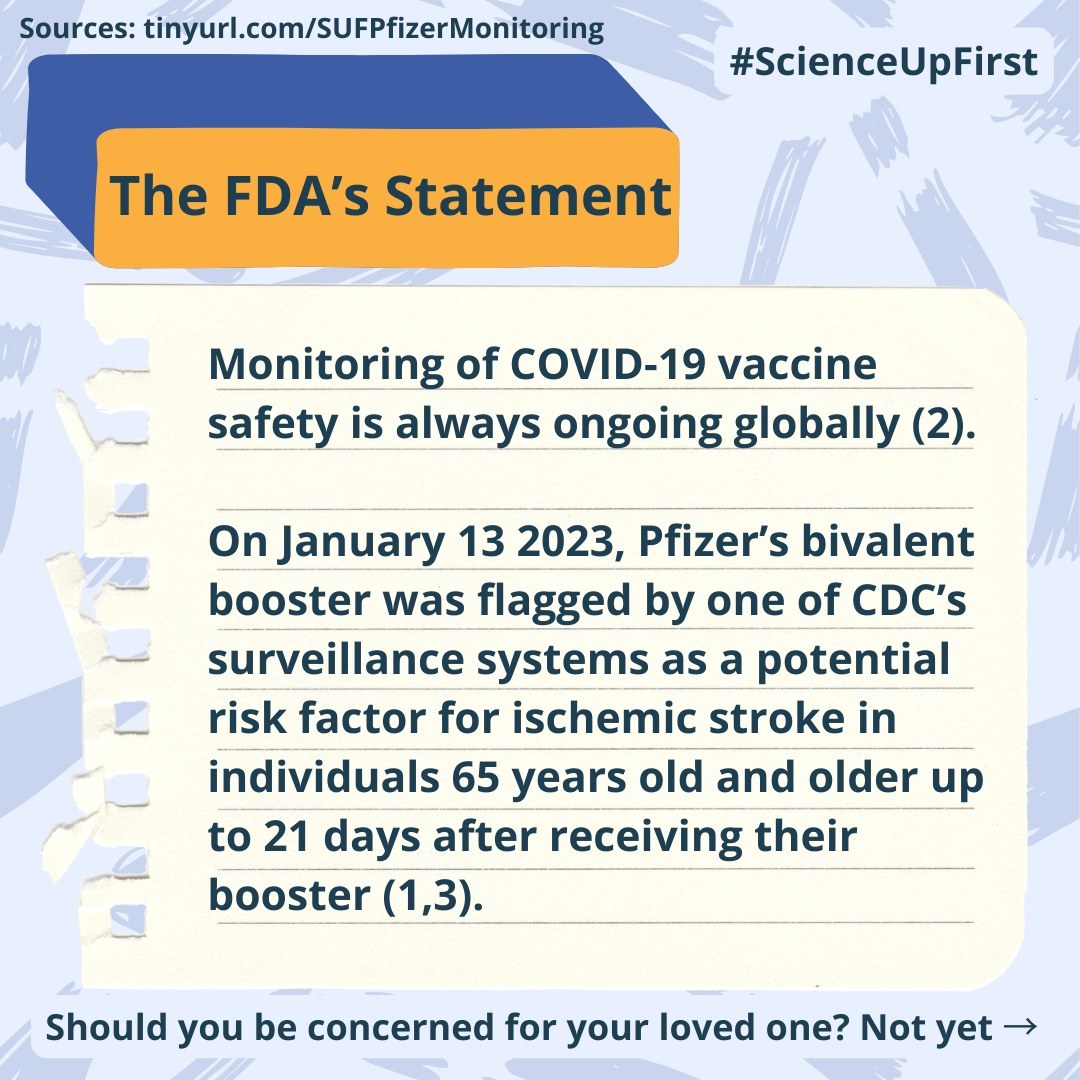
Monitoring of COVID-19 vaccine safety is always ongoing globally (2).
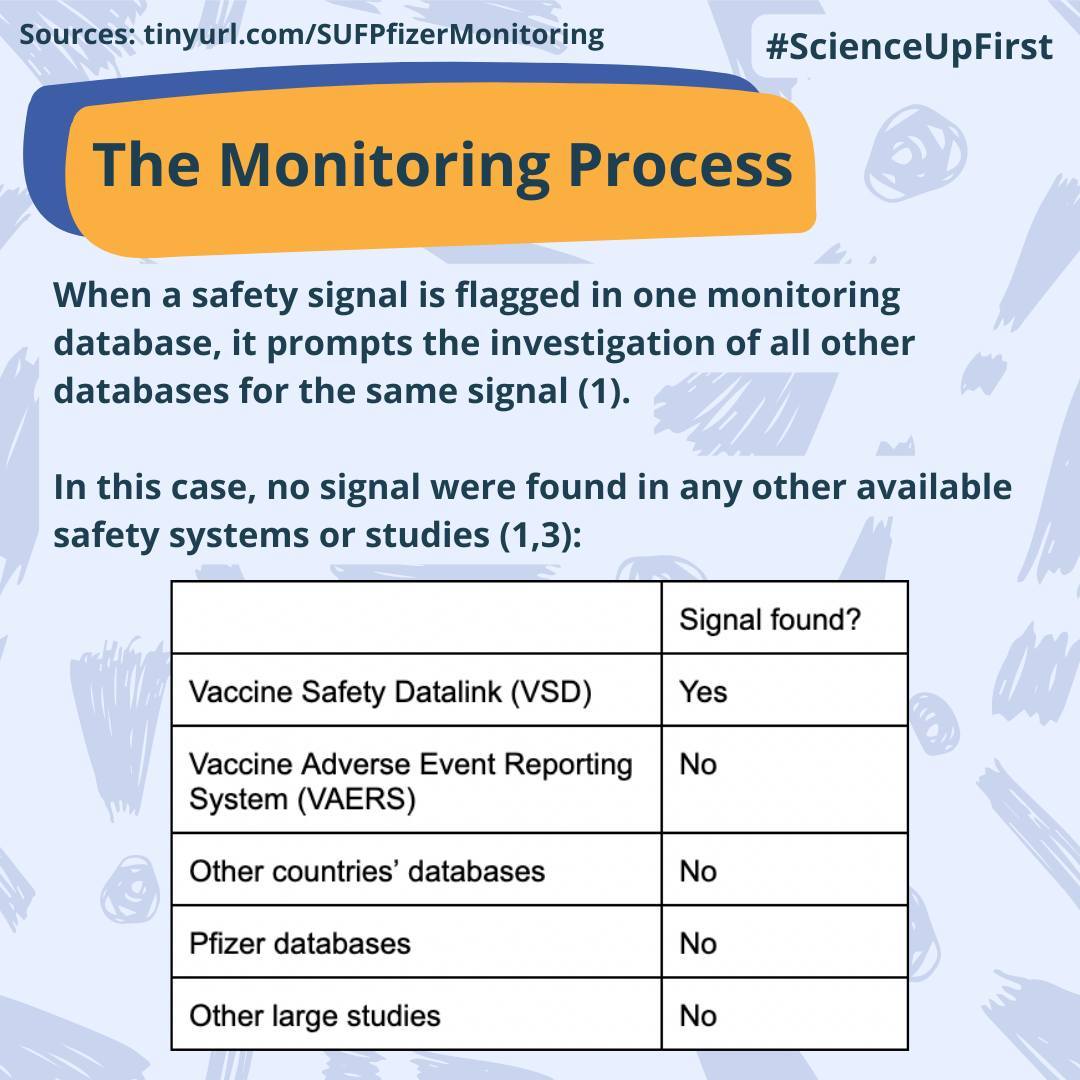
When safety signals are flagged by a monitoring database it prompts further investigation of all other available databases (1).
In this case, the safety signal concerned a potential risk factor for ischemic stroke for anyone 65 years and older in the 21 days following a Pfizer bivalent booster (1).
Symptoms of ischemic stroke includes (5):
- Severe headache
- Numbness or weakness of muscle
- Confusion
- Trouble seeing
- Trouble walking, dizziness, loss of balance or coordination
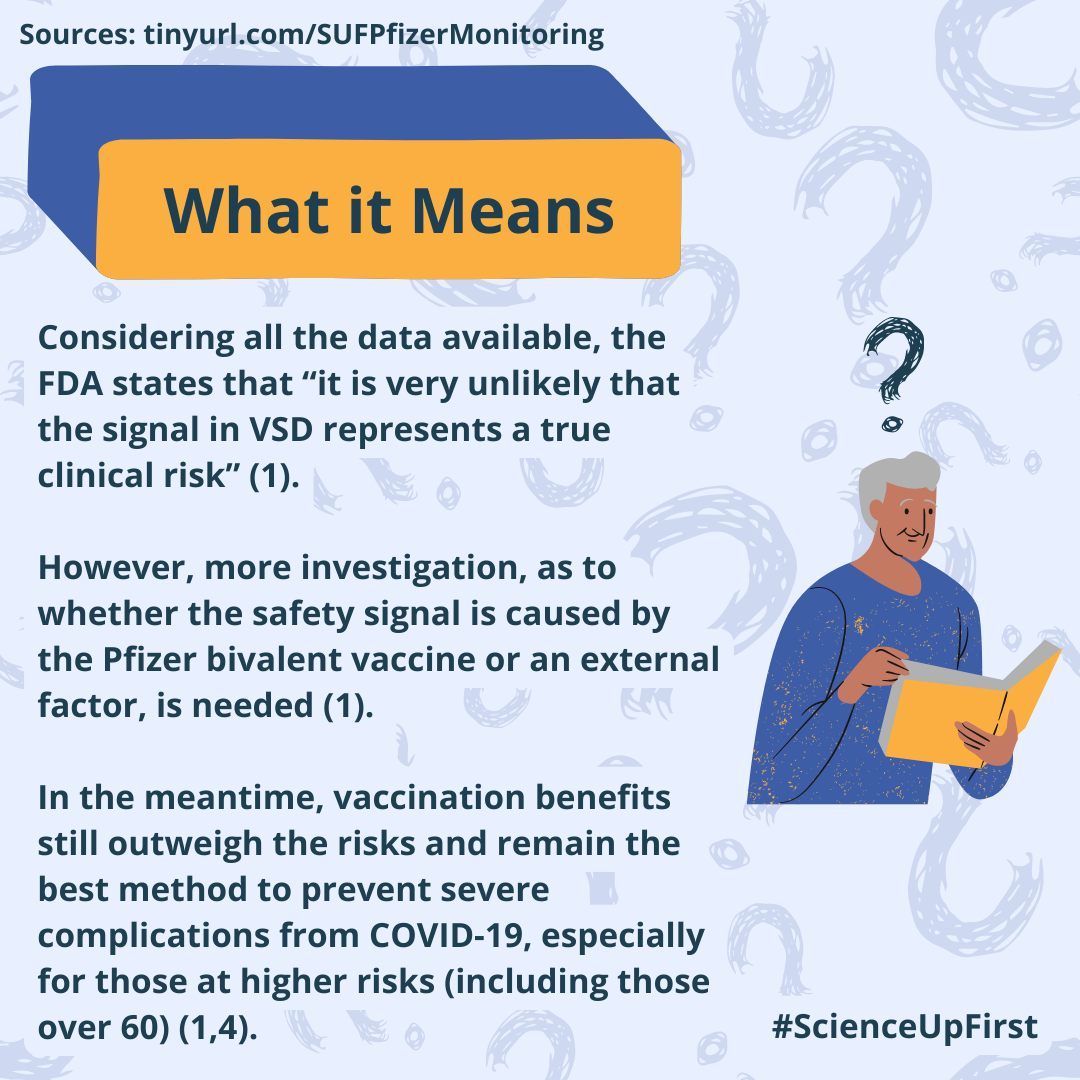
After looking at all the data available over multiple databases it was concluded that there was no true clinical risk associated with the Pfizer bivalent vaccine at this time. However, further investigations are needed to better understand the signal origin (1).
No similar signal was identified in any available monitoring database for Moderna’s bivalent boosters (1).
Share our original Tweet!
Monitoring of COVID-19 vaccine safety is always ongoing globally.
When safety signals are flagged by a monitoring database it prompts further investigation of all other available databases.#ScienceUpFirst
[1/4] pic.twitter.com/U2Sjyq9LJ2
— ScienceUpFirst | LaScienced’Abord (@ScienceUpFirst) January 27, 2023
View our original Instagram Post!
View this post on Instagram
- CDC and FDA Identify Preliminary COVID-19 Vaccine Safety Signal for Persons Aged 65 Years and Older
- Coronavirus disease (COVID-19): Vaccines safety | FR : Maladie à coronavirus 2019 (COVID-19) : sécurité des vaccins
- U.S. FDA, CDC see early signal of possible Pfizer bivalent COVID shot link to stroke
- People who are at risk of more severe disease or outcomes from COVID-19 | FR : Personnes susceptibles de présenter une forme grave de la maladie ou des complications si elles contractent la COVID-19
- Ischemic Stroke: Symptoms & Treatment




